Antibiotic resistance and periodontitis
The New York Times recently reported on infectious bacteria resistant to all antibiotics. (1) Antibiotic resistance (AR) is a natural phenomenon resulting from the widespread use of antibiotics over time. Resistance occurs through bacterial mutations or by acquiring resistance from other bacteria. The Centers for Disease Control and Prevention (CDC) has long raised awareness that this kind of antibiotic resistance is a danger, going so far as to call it the “public health’s ticking time bomb” and asking prescribers to use antibiotics only when other alternatives are not available. The concern is that superbugs with AR will cause untreatable infections.
According to the CDC, bacterial mutations occur in bacteria about one in a million to one in 10 million cell divisions. Bacteria dividing every 20 minutes can form more than 5,000 billion, billion bacteria in 24 hours, and this number of divisions fosters the possibility of mutations. Some mutations can enable the bacteria to produce enzymes that inactivate antibiotics, while other mutations protect the bacteria from the antibiotic—for example, modifying the entry point on the bacteria.
ADDITIONAL READING |Oral-systemic links and tests we never thought about
Resistance is also possible through a bacteriophage in which a virus “infects” the bacteria, and the resistant genetic material is incorporated into the progeny virus that carries the resistance as a part of the virus genetic material. Once the virus infects new bacteria, it may pass along this antibiotic resistance. Bacteria that are resistant to an antibiotic have an increased probability of survival. This natural selection favors the resistant bacteria to then become the predominant species.
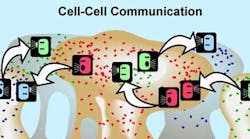
Bacteria can also spread AR through a horizontal mechanism whereby bacteria share the information from one bacteria to the next (figure 1). The sharing of resistance among cells is possible because bacteria are genetically similar. Genetic material can be transferred from one bacteria to another through direct cell contact (conjugation/plasmid exchange) or by incorporation of genetic material through transduction in which bacteria that have resistance die and this genetic material is then incorporated by living bacteria.
Figure 1: ©MSU Center for Biofilm Engineering. Used with permission.
AR is compounded by bacterial antibiotic inactivation. As reported in ScienceDaily, (2) two bacteria living in a biofilm where each is resistant to one antibiotic can protect the other through a mutualistic, interactive phenomenon. Each bacteria makes enzymes that break down the antibiotic, thus removing it from the environment. Bacteria in the biofilm thus benefit from the enzymes of the resistant bacteria.
ADDITIONAL READING |Saving teeth: They must not teach that as the first therapeutic response in dental school
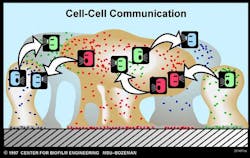
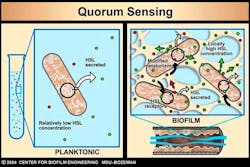
Bacterial communication further advances AR. Bacteria communicate with one another to turn genes on or off, such as resistance genes. Bacteria make quorum-sensing compounds that they release into the environment. Receptors on bacterial cell surfaces or within the bacteria begin to sense the concentration of compounds in the environment. Once the quorum-sensing compounds reach critical mass, bacteria alter their genetic response by exciting or repressing certain genes, including genes for antibiotic resistance (figure 2). This adaptation to environmental changes make it possible for the dominant species at the beginning of biofilm growth to be replaced by another species in a mature biofilm.
Figure 2: ©MSU Center for Biofilm Engineering. Used with permission.
In the human mouth, particularly in the sulcus, modification to the local environment enables the facultative and obligate anaerobes to dominate. The early colonizers in a biofilm are mostly aerobic bacteria. As these are overtaken by facultative anaerobes and eventually by obligate anaerobes, the environment is further modified so that the later colonizers become the dominant species. Obligate anaerobes are especially adaptive, sharing genetic material and AR from one cell to the other, further enabling them to control the environment if antibiotics are introduced into the sulcus.
ADDITIONAL READING | Understanding and managing peri-implant bone loss
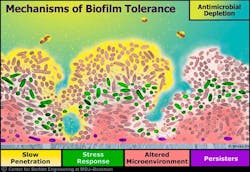
Another factor that favors biofilm is the formation of a matrix (slime layer covering a biofilm) containing compounds that resist antibiotics or increase the resistance of most antibiotics to penetrate the biofilm matrix (figure 3). (3) It has been stated that about a thousand-fold increase of locally delivered antibiotics would be needed to penetrate the matrix of the biofilm sufficiently to control the bacteria. This concentration would be deleterious to the host.
Figure 3:©MSU Center for Biofilm Engineering. Used with permission.
The American Medical Association Guidelines for Chronic Wound Care list the following recommendations for treatment:
2. Wound preparation: debridement and use of adjunctive agents (hyperbaric oxygen)
3. Wound cleansing and maintenance for healing (bacterial balance)
4. Prevention of reoccurrence (4)
Treatment for periodontitis should follow chronic wound care guidelines. Like other biofilm infections, periodontal disease is refractory to antibiotic agents and host defenses. (5) The regular delivery of oxidative and oxygenating agents overcomes the disadvantages of antibiotic therapy and resistance (6-10) by providing care that follows these chronic wound care guidelines.
References
1. Tavernise S, Grady D. Infection raises specter of superbugs resistant to all antibiotics. The New York Times website. nytimes.com/2016/05/27/health/infection-raises-specter-of-superbugs-resistant-to-all-antibiotics.html Published May 26, 2016.
2. Massachusetts Institute of Technology. Teamwork enables bacterial survival: strains of E. coli resistant to one antibiotic can protect other bacteria growing nearby. ScienceDaily website. sciencedaily.com/releases/2016/05/160516181211.htm Published May 16, 2016.
3. Davis, D. Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov. 2003;2(2)114–22.
4. Chronic Wound Care Guidelines. The Wound Healing Society. 2006. woundheal.org/documents/final_pocket_guide_treatment.aspx
5. Schaudinn C, Gorur A, Keller D, Sedghizadeh PP, Costerton JW. Periodontitis: an archetypical biofilm disease. J Am Dent Assoc. 2009;140(8):978–86.
6. Dunlap T, Keller DC, Marshall MV, et al. Subgingival delivery of oral debriding agents: a proof of concept. J Clin Dent. 2011;22(5):149-58.
7. Putt MS, Proskin HM. Custom tray application of peroxide gel as an adjunct to scaling and root planing in the treatment of periodontitis: a randomized, controlled three-month clinical trial. J Clin Dent. 2012;23(2):48–56.
8. Putt MS, Proskin HM. Custom tray application of peroxide gel as an adjunct to scaling and root planing in the treatment of periodontitis: results of a randomized, controlled trial after six months. J Clin Dent. 2013;24(3):100–7.
9. Putt MS, Mallatt ME, Messmann LL, Proskin HM. A six-month clinical investigation of custom tray application of peroxide gel with or without doxycycline as adjuncts to scaling and root planing for treatment of periodontitis. Am J Dent. 2014;27(5):273–84.
10. Cochrane RB, Sindelar B. Case series report of 66 refractory maintenance patients evaluating the effectiveness of topical oxidizing agents. J Clin Dent. 2015;26(4):109–14.