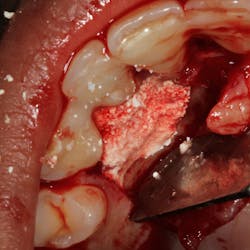
PeriRx LLC, a developer of noninvasive, oral diagnostic technology, has officially launched SaliMark OSCC, a molecular DNA test for the early detection of oral squamous cell carcinoma. The salivary test is now available from the online ordering systems of Henry Schein Dental, Patterson Dental, and Benco Dental.
The test is indicated for use when a visual oral examination (via naked eye or fluorescent device) detects a suspicious lesion or oral mucosal abnormality. SaliMark OSCC’s highly-accurate risk assessment capabilities can help the clinician make a more informed and confident decision on whether to refer the patient to a specialist for a biopsy.
“Although biopsies are the gold standard for determining pathology, they are invasive procedures that come back negative 90% of the time,” explained Swanick. “SaliMark will greatly improve the efficiency and accuracy of any oral cancer exam protocol.”
For more information about PeriRx, LLC or its SaliMark OSCC salivary test for oral squamous cell carcinoma, including scientific data, presentations, videos, peer-reviewed dental journal articles, and product literature, visit www.PeriRx.com, call (610) 544-3500, or send an email to[email protected].
SaliMark OSCC is not yet approved for sale in the state of New York. Regulatory approval process is currently in progress. However, New York dentists who preregister their practices online will be kept on file and notified immediately when SaliMark is approved for sale and use in their state.
Based in Broomall, Pennsylvania, PeriRx, LLC, was founded in 2008. The PeriRx executive team consists of CEO and founder Swanick; Dr. Neil Gottehrer, a practicing dentist; and Dr. Jack Martin, a cardiologist.
PeriRx, LLC, obtained its patented and patent-pending technologies from inventor Dr. David Wong’s firm RNAmeTRIX and the Regents of the University of California at Los Angeles. PeriRx and RNAmeTrix have ongoing agreements that ensure the continuing research and development necessary to advance product development from clinical trial, through regulatory approval, and into commercialization.