Stem cell therapy vs. exosomes: Prospectives for oral tissue regeneration
Key Highlights
- Stem cell benefits are now understood to come largely from exosomes—tiny signaling vesicles that direct inflammation control, angiogenesis, and tissue repair—rather than transplanted cells permanently integrating into tissue.
- Exosomes offer major clinical advantages over stem cells, including improved safety (no tumor risk), reduced immune reaction, easier delivery due to their small size, and greater consistency without relying on cell survival.
- Dentistry is emerging as a strong application area, with growing evidence for exosome-based support in periodontal regeneration, soft tissue healing, and alveolar bone augmentation—including early human case outcomes and promising PRF combinations.
Abstract
Stem cell therapy has proved to be one of the most promising advancements in regenerative medicine and dentistry. The ultimate goal of stem cell therapy is to utilize living cells to fully restore damaged or lost tissue in the body. Decades of scientific research and preclinical trials show the true therapeutic benefit of stem cells comes from the signaling molecules they release, called exosomes. This publication reviews the brief history of stem cell and exosome therapy, highlighting key differences and promise for the future of oral tissue regeneration and regenerative medicine.
Discovery of stem cells
The discovery of stem cells dates back to the early 1960s. Pioneering scientists James Till and Ernest McCulloch conducted a series of experiments injecting bone marrow cells into heavily irradiated mice.1 Ultimately, they showed that the injected bone marrow cells from healthy donor mice were successfully able to rebuild the entire blood-forming system of the irradiated mice, including red blood cells, white blood cells, and platelets. This demonstrates the two key properties of a stem cell: self-renewal, to produce multiple copies of itself, and differentiation, to transform into specialized cell types.
As research continued, a population of nonhematopoietic stem cells was identified within bone marrow and other tissues. These cells showed the capacity to differentiate into multiple mesodermal lineages, including osteoblasts (bone), fibroblasts (connective tissue), chondrocytes (cartilage), and adipocytes (fat). These findings led Arnold Caplan to officially coin the term “mesenchymal stem cells” in 1991.2
Mesenchymal stem cell (MSC) therapy showed much promise for regenerative medicine, dentistry, and orthopedics. However, preclinical and clinical trials revealed several shortcomings. In vivo, MSCs rarely survived long-term or permanently integrated into host tissues. Despite this, therapeutic benefits were still observed.
Subsequent studies demonstrated that the therapeutic effects attributed to mesenchymal stem cells were not driven by their integration or differentiation, but by the extracellular signals they released to existing host cells.3 This discovery led Caplan to propose a conceptual shift—changing the name from mesenchymal stem cells to “medicinal signaling cells” in 2017.4 The primary therapeutic benefit was not the formation of new tissue by the transplanted cells themselves, but their ability to signal and regulate the activity of resident host cells responsible for tissue repair and regeneration. Thus, the regenerative effects historically attributed to stem cells are now understood to be largely mediated by the small extracellular vesicles they secrete, known as exosomes.
Discovery of exosomes
Exosomes are small, secreted vesicles used for cell-to-cell communication. They contain proteins, lipids, and genetic material enclosed by a phospholipid bilayer. When exosomes were first identified in the early 1980s, their significance was fundamentally misunderstood. At that time, they were widely regarded as cellular waste products—extracellular debris released as cells discarded unwanted material.5
As research continued, it became clear that exosomes are not waste particles, but organized vesicles intentionally released by cells carrying powerful bioactive signals. Exosomes were shown to influence key biological processes such as inflammation, angiogenesis, and tissue repair in recipient cells.6
Many positive outcomes originally attributed to stem cells could be explained by the signaling effects of exosomes released by these cells.7 Accordingly, regenerative medicine has shifted its focus toward exosomes, which represent a more promising treatment modality that overcomes the limitations of stem cell-based therapies. A detailed comparison of stem cells and exosomes reveals the promise of exosome therapy.
Stem cells vs. exosomes
Cell vitality
Perhaps the most fundamental difference between stem cells and exosomes is that stem cells are living cells, while exosomes are not. This means that the treatment of stem cell therapy largely relies on the vitality of these cells after transplantation. Some studies have shown that up to 90% of transplanted stem cells do not survive within days after transplantation, due to apoptosis, reactive oxygen species, and host immunity.8 Conversely, exosomes are nonliving vesicles. Their effects rely entirely on the delivery of bioactive factors to preexisting host cells, bypassing the shortcomings associated with cell survival.
Safety and consistency
Exosomes consistently show higher levels of safety and fewer side effects than stem cell-based therapy.9 Additionally, living stem cells express major histocompatibility complex class I (MHC I) receptors. Their expression can trigger the host immune system and clearance, particularly with allogenic stem cells. Because stem cells are living and capable of proliferation and differentiation, they also carry a potential risk of tumor formation, whereas exosomes, as nonliving vesicles, eliminate this risk entirely.10
Size
Exosomes range in size from 30 nm to 150 nm in diameter, while stem cells measure roughly 100–1,000 times larger at 15–25 μm. The smaller, more stable structure of exosomes allows for easier delivery, penetration into tissues, and storage.11
Stability and storage
Stem cells require careful handling to maintain viability.12 Autologous stem cells must be harvested, expanded, and often cryopreserved prior to use, while allogeneic stem cells often struggle with survival. Exosomes, on the other hand, are inherently more stable, can be stored in a freezer for extended periods, and retain their biological activity after freeze-thaw cycles.13 This stability makes exosomes a more practical and scalable therapeutic option for clinical practice.
Conclusion
Exosome-based therapy represents a next-generation approach to regenerative medicine that builds upon the foundational discoveries of stem cell research, while overcoming many of its practical and biological limitations. This paradigm shift shows promise for a safe, more predictable, and clinically adaptable form of treatment.
Within dentistry, growing evidence supports the application of exosomes for treatment of periodontal disease, soft tissue grafting, and alveolar bone regeneration. Preclinical studies demonstrate that stem cell-derived exosomes reduce local inflammation while enhancing osteogenesis and periodontal healing in animal models.14,15
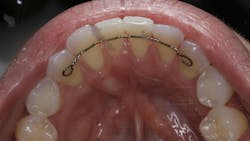
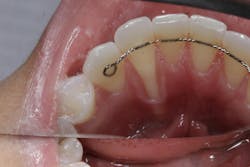
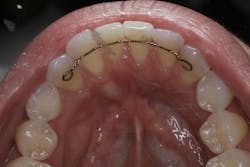
We are amidst an incredibly exciting time in the field of oral tissue regeneration. More recently, a published case report utilizing exosome-mediated alveolar bone augmentation shows impressive clinical and histologic outcomes for the first time in a human subject.16 Another recent study looked at the use of exosomes mixed with platelet-rich fibrin (PRF) for root recession coverage and showed the exosome PRF group had root coverage equivalent to connective tissue grafting studies but without the side effects of harvesting autogenous tissue (figures 1 and 2).17
Collectively, these findings position exosome-based therapies as a highly promising, cell-free regenerative strategy for improving outcomes across periodontal practice and implant dentistry.
Editor’s note: This article originally appeared in Perio-Implant Advisory, a chairside resource for dentists and hygienists that focuses on periodontal- and implant-related issues. Read more articles and subscribe to the newsletter.
References
- Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiation Res. 1961;14:213-222.
- Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641-650. doi:10.1002/jor.1100090504
- Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9(1):11-15. doi:10.1016/j.stem.2011.06.008
- Caplan AI. Mesenchymal stem cells: time to change the name! Stem Cells Transl Med. 2017;6(6):1445-1451. doi:10.1002/sctm.17-0051
- Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation: association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262:9412-9420.
- Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373-383. doi:10.1083/jcb.201211138
- Malik S, Muhilan Y, Nordin F, et al. Stem cell derived exosome trilogy: an epic comparison of human MSCs, ESCs and iPSCs. Stem Cell Res Ther. 2025;16:318. doi:10.1186/s13287-025-04440-0
- Salazar-Noratto GE, Luo G, Denoeud C, Padrona M, Moya A, Bensidhoum M, Bizios R, Potier E, Logeart-Avramoglou D, Petite H. Understanding and leveraging cell metabolism to enhance mesenchymal stem cell transplantation survival in tissue engineering and regenerative medicine applications. Stem Cells. 2020;38(1):22-33. doi:10.1002/stem.3079
- Malekpour K, Hazrati A, Zahar M, et al. The potential use of mesenchymal stem cells and their derived exosomes for orthopedic diseases treatment. Stem Cell Rev Rep. 2021;18(3):933-951. doi:10.1007/s12015-021-10185-z
- Zhang K, Cheng K. Stem cell-derived exosome versus stem cell therapy. Nat Rev Bioeng. 2023;1:608-609. doi:10.1038/s44222-023-00064-2
- Rezabakhsh A, Sokullu E, Rahbarghazi R. Applications, challenges and prospects of mesenchymal stem cell exosomes in regenerative medicine. Stem Cell Res Ther. 2021;12(1):521. doi:10.1186/s13287-021-02596-z
- Tan F, Li X, Wang Z, Li J, Shahzad K, Zheng J. Clinical applications of stem cell-derived exosomes. Signal Transduct Target Ther. 2024;9(1):17. doi:10.1038/s41392-023-01704-0
- Lai RC, Yeo RWY, Lim SK. Mesenchymal stem cell exosomes. Semin Cell Dev Biol. 2015;40:82-88. doi:10.1016/j.semcdb.2015.03.001
- Qiao X, Tang J, Dou L, et al. Dental pulp stem cell-derived exosomes regulate anti-inflammatory and osteogenic effects in periodontal ligament stem cells and promote the repair of experimental periodontitis in rats. Int J Nanomedicine. 2023;18:4683-4703. doi:10.2147/IJN.S420967
- Wu J, Chen L, Wang R, et al. Exosomes secreted by stem cells from human exfoliated deciduous teeth promote alveolar bone defect repair through the regulation of angiogenesis and osteogenesis. ACS Biomater Sci Eng. 2019;5(7):3561-3571. doi:10.1021/acsbiomaterials.9b00607
- Estrin NE, Farshidfar N, Ahmad P, et al. Exosome-mediated alveolar ridge augmentation: a first human case report with histology. Int J Periodontics Restorative Dent. 2026;46(1):30-41. doi:10.11607/prd.7567
- Froum S, Estrin NE, Ahmad P, Farshidfar N, Miron RJ. The feasibility of exosome-enriched platelet-rich fibrin (PRF) for the treatment of gingival recessions: a case series of 27 patients. Oral Health Prev Dent. 2026;24:48-57. doi:10.3290/j.ohpd.c_2394
About the Author
Stephen Kelleher, DMD
Stephen Kelleher, DMD, is a periodontist practicing in the greater New York City area, originally from Pittsburgh, Pennsylvania. He earned his DMD from Case Western Reserve University in 2022 and completed his periodontics and implant dentistry training at New York University in 2025. Dr. Kelleher has received several awards recognizing his excellence in periodontics and regenerative dentistry. He is dedicated to evidence-based, patient-centered care with a focus on periodontal therapy, oral tissue regeneration, and implant surgery.

Scott Froum, DDS
Editorial Director
Scott Froum, DDS, a graduate of the State University of New York, Stony Brook School of Dental Medicine, is a periodontist in private practice at 1110 2nd Avenue, Suite 305, New York City, New York. He is the editorial director of Perio-Implant Advisory and serves on the editorial advisory board of Dental Economics. Dr. Froum, a diplomate of both the American Academy of Periodontology and the American Academy of Osseointegration, is in the fellowship program at the American Academy of Anti-aging Medicine, and is a volunteer professor in the postgraduate periodontal program at SUNY Stony Brook School of Dental Medicine. He is a trained naturopath and is the scientific director of Meraki Integrative Functional Wellness Center. Contact him through his website at drscottfroum.com or (212) 751-8530.