COVID-19 and the problem with dental aerosols
Updated April 13, 2020
Background
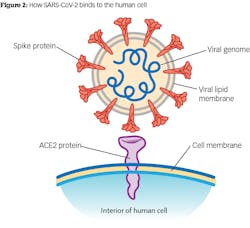
A novel human coronavirus—now named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)—emerged from Wuhan, China, in late 2019 and is causing a pandemic.1 Coronaviruses are enveloped RNA viruses that affect animals and humans.2 Coronavirus particles range from 60 to 140 nanometers (0.06 to 0.14 micrometers), with an average of 0.125 micron, and have distinctive spikes of nine to 12 nanometers that give the appearance of “coronas” around the sun (figure 1). Cell death is observed 96 hours after inoculation on surface layers of human airway epithelial cells.2
Currently, there are six coronavirus species that cause human disease. Four of them—229E, OC43, NL63, and HKU1—often result in symptoms of the common cold.3 The other two strains—severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV)—are zoonotic (originate from animals and cross over to humans), more serious, and sometimes linked to fatal illness.4
SARS-CoV-1 was the causal agent of the severe acute respiratory syndrome outbreaks in 2002 and 2003 in Guangdong Province, China.5 During this outbreak, approximately 8,098 patients were affected with 774 deaths, resulting in a mortality rate of 9%. This rate was much higher in elderly individuals, with mortality rates approaching 50% in those over age 60. Transmission of SARS-CoV-1 was relatively inefficient because it spread only through direct contact with infected individuals; once an individual exhibited symptoms, the virus spread. The outbreak was largely contained because it was easy to identify those individuals who were capable of spreading the disease. A few cases of super-spreading events occurred whereby individuals with higher viral loads and the ability to aerosolize the virus were able to infect multiple people. As a result of the relatively inefficient transmission of SARS-CoV-1, its outbreak was controllable through the means of quarantining individuals in households and health-care centers.6
Aerosol particle transmission
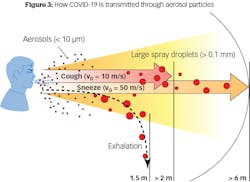
Particles are classified based on size: coarse particles are 2.5–10 microns, fine particles are less than 2.5 microns, and ultrafine particles are less than 0.1 micron. The nose typically filters air particles above 10 microns. If a particle is less than 10 microns, it can enter the respiratory system. If it is less than 2.5 microns, it can enter the alveoli. A particle less than 0.1 micron, or an ultrafine particle like the COVID-19 virus, can enter the bloodstream and target organs such as the heart and brain. The current scientific consensus is that most transmission via respiratory secretions happens in the form of large respiratory droplets rather than small aerosols. Droplets are often heavy enough that they do not travel very far; instead, they fall from the air after traveling up to six feet (figure 3).
• The virus is viable up to 72 hours after application to plastic and stainless steel surfaces.
• The virus is viable up to 24 hours on cardboard surfaces.
• The virus is viable up to nine hours on copper surfaces.
• The virus is viable in suspended aerosols up to three hours.
Viral dosimetry and dental considerations
Whenever a new virus emerges, the question needs to be asked if there is a dose-dependent response between viral load contact and severity of the disease. In other words, does the number of viral particles a patient initially encounters, or repeated dosing, determine the severity of the symptoms? One study reported that viral loads in nasopharyngeal swabs from a group of patients with severe COVID-19 were 60 times higher on average than the viral loads seen among patients with a mild form of the disease.9
If this is the case, dental aerosolization may pose an additional threat. Does a patient who has viral particles confined to the nasopharyngeal area become susceptible to aerosol aspiration into the lungs, leading to increased disease severity? This question was inspired by and based on the work of Bruce L Davidson, MD, MPH—a pulmonary physician and researcher in Seattle, expert in respiratory transmission of infection, former president of the National Tuberculosis Controllers Association, and member of the HHS Secretary’s Advisory Council for the Elimination of Tuberculosis—who has extensively looked at aspirational types of pneumonia.15 According to Dr. Davidson, "This very real possibility can be easily diminished by reducing biofilm viral load in the mouth and pharynx region with 1.5% peroxide for 60 seconds, thereby reducing viral load and basically disinfecting the throat. Peroxide drops cornavirus replication by >4 logs. These types of debridement controls are often overlooked." In addition, Dr. Davidson states that nose-covering filters and devices are simple and effective. Of course, well-designed controlled studies are needed to further this research and recommendation.
Dental aerosolization
Dentists who treat patients using aerosolization are at an extremely dangerous risk of inoculation of themselves, their dental assistants, other office staff members, and reinoculation of the patients. Most risk occurs from splatter and droplet transmission to the midface of the dentist and assistant, as well as the nasal area of the patient.10 In addition, periodontal treatment has a much higher incidence of droplet transmission than prosthetic treatment.11 Ultrasonic and sonic transmission during nonsurgical procedures had the highest incidence of particle transmission, followed by air polishing, air/water syringe, and high-speed handpiece aerosolization.12 One study found that ultrasonic instrumentation can transmit 100,000 microbes per cubic foot with aerosolization of up to six feet, and, if improper air current is present, microbes can last anywhere from 35 minutes to 17 hours.13
Because of these inherent dangers to dentists, team members, and patients, the Occupational Safety and Health Act (OSHA) just released a new report called “Guidance on Preparing Workplaces for COVID-19.”14 This document categorizes occupational risk as very high, high, medium, and lower risk. The occupations that are involved with aerosol production fall into the category of very high risk, according to OSHA.
Since dentistry is in the very-high-risk category, the section “Implement Workplace Controls, Engineering Controls” recommends that dental practices install negative-pressure rooms or airborne infection isolation rooms for operatories in which procedures involving aerosol will be performed. In addition, recommendations for the dentist and staff working in areas of direct contact with aerosols include wearing the following personal protective equipment (PPE) masks: “Other types of acceptable respirators include: a R/P95, N/R/P99, or N/R/P100 filtering facepiece respirator; an air-purifying elastomeric (e.g., half-face or full-face) respirator with appropriate filters or cartridges; powered air-purifying respirator (PAPR) with high-efficiency particulate arrestance (HEPA) filter; or supplied air respirator (SAR).”14Conclusion
Many changes in infection control procedures and the associated dental armamentaria can be expected to arise in the post-COVID-19 world of dentistry. The extent and severity of change will be dictated by evidence and research into the best and safest practices. Prior to mandating change that will involve an extreme financial and architectural change of the current dental office, research should be conducted that evaluates current available practices, methodology, and instrumentation that can mitigate/obviate the risk of transmission, while being financially and practically expeditious.
References
1. Coronavirus disease 2019 (COVID-2019) situation report—32. World Health Organization. February 21, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200221-sitrep-32-covid-19.pdf
2. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-733. doi:10.1056/NEJMoa2001017
3. Su S, Wong G, Shi W, et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24(6):490-502. doi:10.1016/j.tim.2016.03.003
4. Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17(3):181-192. doi:10.1038/s41579-018-0118-9
5. Drosten C, Günther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1967-1976. doi:10.1056/NEJMoa030747
6. Peiris JSM, Yuen KY, Osterhaus ADME, Stohr K. The severe acute respiratory syndrome. N Engl J Med. 2003;349(25):2431-2441. doi:10.1056/NEJMra032498
7. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. Published online February 21, 2020. doi:10.1001/jama.2020.2565
8. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. Published online March 17, 2020. doi:10.1056/NEJMc2004973
9. Verity R, Okell LC, Dorigatti I, et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. Published online March 30, 2020. doi:10.1016/S1473-3099(20)30243-7
10. Nejatidanesh F, Khosravi Z, Goroohi H, Badrian H, Savabi O. Risk of contamination of different areas of dentist’s face during dental practices. Int J Prev Med. 2013;4(5):611-615.
11. Williams GH, Pollok NL, Shay DE, Barr CE. Laminar air purge of microorganisms in dental aerosols: prophylactic procedures with the ultrasonic scaler. J Dent Res. 1970;49(6):1498-1504. doi:10.1177/00220345700490065701
12. Harrel SK, Molinari J. Aerosol and splatter in dentistry: a brief review of the literature and infection control implications. J Am Dent Assoc. 2004;135(4):429-437. doi:10.14219/jada.archive.2004.0207
13. Miller RL. Characteristics of blood-containing aerosols generated by common powered dental instruments. Am Ind Hyg Assoc J. 1995;56(7):670-676. doi:10.1080/15428119591016683
14. Guidance on preparing workplaces for COVID-19. US Department of Labor. Occupational Safety and Health Administration. 2020. https://www.osha.gov/Publications/OSHA3990.pdf
15. Davidson BL. Doctor: How to reduce your vulnerability to coronavirus -- when sleeping. CNN. Updated March 13, 2020. https://www.cnn.com/2020/03/12/opinions/coronavirus-vulnerability-while-sleeping-bruce-davidson/index.html
Author's note: Special thanks to Dr. Bruce L. Davidson for the consult on this article.
Editor’s note: This article originally appeared in Perio-Implant Advisory, a newsletter for dentists and hygienists that focuses on periodontal- and implant-related issues. Perio-Implant Advisory is part of the Dental Economics and DentistryIQ network. To read more articles, visit perioimplantadvisory.com, or to subscribe, visit dentistryiq.com/subscribe.
About the Author

Scott Froum, DDS
Editorial Director
Scott Froum, DDS, a graduate of the State University of New York, Stony Brook School of Dental Medicine, is a periodontist in private practice at 1110 2nd Avenue, Suite 305, New York City, New York. He is the editorial director of Perio-Implant Advisory and serves on the editorial advisory board of Dental Economics. Dr. Froum, a diplomate of both the American Academy of Periodontology and the American Academy of Osseointegration, is in the fellowship program at the American Academy of Anti-aging Medicine, and is a volunteer professor in the postgraduate periodontal program at SUNY Stony Brook School of Dental Medicine. He is a trained naturopath and is the scientific director of Meraki Integrative Functional Wellness Center. Contact him through his website at drscottfroum.com or (212) 751-8530.

Michelle Strange, MSDH, RDH
Michelle Strange, MSDH, RDH, has more than two decades of dental expertise, beginning as a dental assistant and completing a bachelor’s degree in health science from the Medical University of South Carolina and a master’s in dental hygiene education from the University of Bridgeport. She continues to invest in ongoing education, gaining certifications such as her Certificate in Dental Infection Prevention and Control. Her community and global endeavors demonstrate her passion for dentistry, from volunteer work to worldwide missions. She is the owner of Level Up Infection Prevention, MichelleStrangeRDH, a practicing dental hygienist, and was the cofounder and the fire and energy that made A Tale of Two Hygienists podcast.
Updated October 10, 2022