Salivary diagnostics for COVID-19 prove as reliable as nasopharyngeal swab in new study
A new noninvasive COVID-19 test utilizing salivary diagnostics has recently been released for public and point-of-care use. Sample collection is very easy, and the test is less prone to operator error. The question is, are salivary diagnostics as reliable as the gold standard nasopharyngeal swabs? A new prospective study in the Journal of Clinical Microbiology looked to answer that question.1
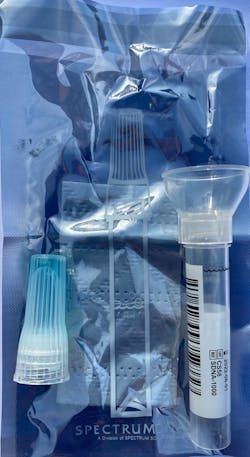
Saliva tests (figure 1) have definite benefits over traditional nasopharyngeal swabs:
- Health-care workers do not need to be exposed to patients upon collection.
- These tests are noninvasive and simple to use.
- The patient does not have to go to a clinic with sick patients to be tested.
- Lab results can take 48–72 hours depending on demand.
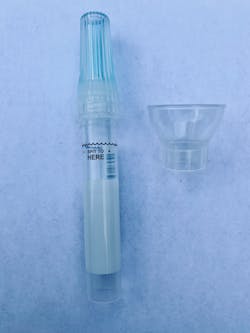
- The patient expectorates (spits) into a collection tube (figure 2) up to a required volume.
- The patient screws on a buffer solution and mixes the saliva and buffer (figure 3).
- The patient places the sample in an envelope and mails it overnight to the lab (figure 4).
Mass implementation of a COVID-19 test that is accurate, easy to use, and efficient with test results can assist both health care and public health officials in ending the COVID-19 pandemic in an expedited manner.
Related articles
- Can periodontal disease be a contributing factor for COVID-19 severity?
- Downloadable COVID-19 screening form for dental patients
- The importance of periodontal treatment in post–COVID-19 dentistry
- CDC revises guidance on isolation after COVID-19–positive test
- Uncontrolled inflammation: The real problem with COVID-19 severity
- COVID-19 serology tests and the role of the dental office
Editor’s note: This article originally appeared in Perio-Implant Advisory, a newsletter for dentists and hygienists that focuses on periodontal- and implant-related issues. Perio-Implant Advisory is part of the Dental Economics and DentistryIQ network. To read more articles, visit perioimplantadvisory.com and subscribe at this link.
Reference
- Hanson KE, Barker AP, Hillyard DR, et al. Self-collected anterior nasal and saliva specimens versus healthcare worker-collected nasopharyngeal swabs for the molecular detection of SARS-CoV-2. J Clin Microbiol. 2020;JCM.01824-20. Published online: August 12, 2020. doi:10.1128/JCM.01824-20
About the Author

Scott Froum, DDS
Editorial Director
Scott Froum, DDS, a graduate of the State University of New York, Stony Brook School of Dental Medicine, is a periodontist in private practice at 1110 2nd Avenue, Suite 305, New York City, New York. He is the editorial director of Perio-Implant Advisory and serves on the editorial advisory board of Dental Economics. Dr. Froum, a diplomate of both the American Academy of Periodontology and the American Academy of Osseointegration, is a volunteer professor in the postgraduate periodontal program at SUNY Stony Brook School of Dental Medicine. He is a PhD candidate in the field of functional and integrative nutrition. Contact him through his website at drscottfroum.com or (212) 751-8530.