Saving teeth: Changing the hopeless prognosis with new technology
Classically, a tooth that was affected with greater than 50% bone loss was given a questionable to hopeless prognosis. Dr. Scott Froum explains how new developments in laser and osteoblastic promotive technologies have surmounted historical restrictions of tissue repair/regeneration around diseased teeth and changed expected outcomes in periodontal treatment for patients interested in preserving their natural dentition.
RECENTLY, A COMBINED EFFORT by the American Academy of Periodontology (AAP) and the European Federation of Periodontology (EFP) resulted in the Proceedings of the World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. These changes for the new classification of periodontal and peri-implant diseases included labeling tooth and dental implant disease as well as staging (I–IV), distribution (generalized versus localized), rate of progression, and anticipated response to treatment. (1) Although the new classification was comprehensive in discussion as far as the justification for labeling types of disease, very little was said regarding the treatment for disease. In addition, what was mentioned about anticipated response to treatment for disease was based on classical periodontal treatment methods.
Classically, a tooth that was affected with greater than 50% bone loss was given a questionable to hopeless prognosis. (2) In addition, if the tooth was a multirooted tooth and exhibited furcation involvement, the tooth was further downgraded toward the hopeless category and extraction treatment. Periodontal treatment, even in advanced bone loss situations, has been shown to increase tooth survival rates in the literature with the conclusion that only a small group of study subjects lost the majority of the teeth. (3) Most of the tooth loss was due to failed endodontic procedures and/or noncompliance with recommended hygiene intervals.
Although the classic literature has shown that periodontal treatment works, the threshold for tooth extraction in favor of implant therapy remains low. One study suggests that anterior incisors with 50% bone loss, premolars with 48% bone loss, and molars with 42% bone loss are likely to be extracted instead of saved with periodontal therapy. (4) Furthermore, advances in both laser technology and growth factor technology can regenerate or repair bone defects around teeth that are most often slated for extraction. New developments in both laser and osteoblastic promotive technologies have changed expected outcomes in the field of periodontal treatment. This article explains historical limitations of tissue repair/regeneration around diseased teeth and how technology has been able to surmount those restrictions for patients interested in preserving their natural dentition.
There are many risk factors and risk indicators used in the academic and clinical world that determine the prognosis of teeth and their anticipated survival outcome with regard to periodontal therapy. (5) Clinically, the history of tooth loss, mobility, percent of radiographic bone loss, and clinical attachment level continue to be the most useful factors when determining tooth prognosis. (6) In terms of response to treatment, the extent of disease around the tooth/teeth in question (probing depth, furcation involvement, mobility, type of bone loss) as well as patient factors (medical history, smoking status, compliance with hygiene) also may sway a clinician’s choice as to whether to treat the disease or extract the tooth.
In this author’s opinion, there are three main questions that must be answered to determine anticipated success of treatment:
1. Can the depth of the defect (bottom portion of the tooth root) be accessed and completely detoxified to allow for tissue repair/regeneration?
2. How much osteoblastic potential does the defect have that will allow for repair/regeneration? This potential is usually determined by how many bone walls remain around the diseased tooth.
3. Is the patient compliant with recommended home hygiene and maintenance intervals? (7)
Let us now examine how these three questions and new technology intersect.
Classic studies have shown how our ability as clinicians to fully decontaminate the tooth root decrease as probing depth and bone loss increase. The following chart is from one study that compares the effectiveness of scaling and root planing in decontaminating the root surface to increases in probing depth (table 1): (8)
Additionally, even after multiple episodes of scaling and root planing, additional calculus removal showed no difference between single attempts versus multiple attempts. (9) In other words, even experienced clinicians cannot remove calculus around deep defects without proper access. However, even when access is possible, some defects are too deep to clean with conventional instrumentation. If bacteria and calculus are left, tissue repair/regeneration can be impeded. The following chart is from one study that evaluated the effectiveness of scaling versus flap surgery when decontaminating the root as it relates to the increase in probing depth (table 2): (10)
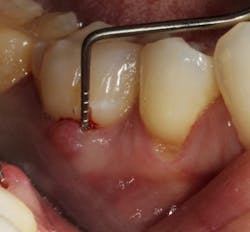
In other words, even experienced clinicians who access a root surface with flap surgery are sometimes limited in the amount of detoxification that can occur due to the depth of the defect and conventional instrumentation. (11) Further limitations with mechanical access to defects can come in the form of furcations with multirooted teeth. The diameter of the entrance to root furcation is usually smaller than the average tip of a curette, which can make access difficult (figures 1 and 1a). Molars with bone loss that includes furcations are often downgraded in prognosis due to this access difficulty. (12)
Figure 1: Molar with periodontal abscess due to furcation involvement
Figure 1a: Deep Class II furcation with an entrance smaller than the diameter of a curette tip
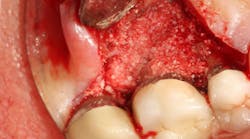
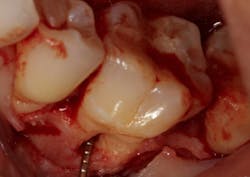
The advent of laser technology, either as an adjunct to surgical therapy or as a monotherapy, has been met with mixed results in the literature. (13) Recently, a new 9.3-micron CO2 laser (Solea from Convergent Dental) with the ability to cut both hard and soft tissue with efficiency was introduced to the dental market. This laser can penetrate deep defects with enough power to both thoroughly detoxify root surfaces and remove fibrous tissue, enhancing tissue repair (figure 2). (14) The spot size of the focal laser beam can also be controlled to a range of .25 mm to 1.25 mm so that the beam can be directed into furcation entrances. This allows previously difficult furcal defects to be detoxified prior to regenerative therapy. Because of this enhanced ability to detoxify, this laser has been able to change the prognosis of once-hopeless teeth (figure 3) and allow for tissue regeneration (figure 4) instead of extraction.
Figure 2: Solea laser with a beam size of .5 mm used to detoxify furcation. This beam can penetrate deep into the furcation.
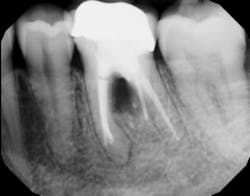
Figure 3: Tooth that would normally need to be extracted and replaced with an implant
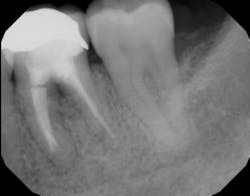
Figure 4: Same tooth one year later after laser detoxification and periodontal regeneration with growth-stimulating factors
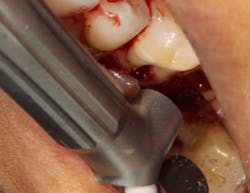
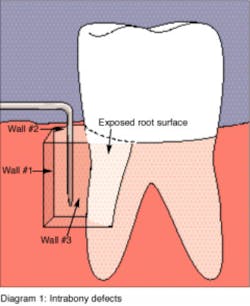
Another advancement in the field of periodontal regenerative medicine has been due to the use of growth factors, proteins, and stem cells. The ability to repair/regenerate a diseased tooth is often dependent upon the extent of the defect, namely the number of bone walls that are left surrounding the tooth. The more bone walls a tooth has left around it (maximum of four), the more blood supply, containment, and space maintenance your graft material will have (figure 5). Bone defects that have three to four walls missing are the hardest to repair and may be slated for extraction (figure 6). By adding growth-stimulating factors to your regular bone grafts (figure 7), an increase in osteopromotive potential occurs that can have the ability to overcome a deficiency of bone walls. (15) Simply stated, your bone graft can become alive, and teeth that were once unable to be repaired now have a chance.
Figure 5: This graphic shows the type of walls remaining around a tooth affected with periodontal disease. This image is from RDH magazine.
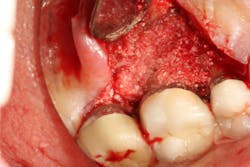
Figure 6: Molar tooth with three walls missing around the palatal root. This tooth would normally be extracted.
Figure 7: Same tooth undergoing periodontal regeneration surgery with anorganic bovine bone (Bio-Oss Collagen, Geistlich Biomaterials) and platelet-derived growth factor (PDGF)
In summary, advancements in technology have given clinicians the ability to access difficult bone defects and allow for the detoxification of bacteria. In addition, challenging defects with few remaining bony walls can now be overcome with increased osteoblastic activity of bone grafts with the addition of growth-stimulating factors. The combination of these two advancements have allowed clinicians to save teeth that were once planned for extraction.
References
1. Caton JG, Armitage G, Berglundh T, et al. A new classification scheme for periodontal and peri‐implant diseases and conditions – Introduction and key changes from the 1999 classification. J Clin Periodontol. 2018;45(suppl 20):S1-S8. doi:10.1111/jcpe.12935.
2. Ioannou AL, Kotsakis GA, Hinrichs JE. Prognostic factors in periodontal therapy and their association with treatment outcomes. World J Clinical Cases. 2014;2(12):822-827. doi:10.12998/wjcc.v2.i12.822.
3. Hirschfeld L, Wasserman B. A long-term survey of tooth loss in 600 treated periodontal patients. J Periodontol. 1978;49(5):225-237. doi:10.1902/jop.1978.49.5.225.
4. Splieth C, Giesenberg J, Fanghanel J, Bernhardt O, Kocher T. Periodontal attachment level of extractions presumably performed for periodontal reasons. J Clin Periodontol. 2002;29(6):514-518. doi:10.1034/j.1600-051X.2002.290607.x.
5. Albandar JM. Global risk factors and risk indicators for periodontal diseases. Periodontol 2000. 2002;29(1):177-206.
6. McGuire MK, Nunn ME. Prognosis versus actual outcome. II. The effectiveness of clinical parameters in developing an accurate prognosis. J Periodontol. 1996;67(7):658-665. doi:10.1902/jop.1996.67.7.658.
7. Wilson TG Jr, Glover ME, Malik AK, Schoen JA, Dorsett D. Tooth loss in maintenance patients in a private periodontal practice. J Periodontol. 1987;58(4):231-235. doi:10.1902/jop.1987.58.4.231.
8. Rabbani GM, Ash MM Jr, Caffesse RG. The effectiveness of subgingival scaling and root planing in calculus removal. J Periodontol. 1981;52(3):119-123. doi:10.1902/jop.1981.52.3.119.
9. Anderson GB, Palmer JA, Bye FL, Smith BA, Caffesse RG. Effectiveness of subgingival scaling and root planing: single versus multiple episodes of instrumentation. J Periodontol. 1996;67(4):367-373. doi:10.1902/jop.1996.67.4.367.
10. Caffesse RG, Sweeney PL, Smith BA. Scaling and root planing with and without periodontal flap surgery. J Clin Periodontol. 1986;13(3):205-210.
11. Brayer WK, Mellonig JT, Dunlap RM, Marinak KW, Carson RE. Scaling and root planing effectiveness: the effect of root surface access and operator experience. J Periodontol. 1989;60(1):67-72. doi:10.1902/jop.1989.60.1.67.
12. Svärdström G, Wennström JL. Periodontal treatment decisions for molars: an analysis of influencing factors and long-term outcome. J Periodontol. 2000;71(4):579-585. doi:10.1902/jop.2000.71.4.579.
13. Romanos G. Current concepts in the use of lasers in periodontal and implant dentistry. J Indian Soc Periodontol. 2015;19(5):490-494. doi:10.4103/0972-124X.153471.
14. Fan K, Bell P, Fried D. Rapid and conservative ablation and modification of enamel, dentin, and alveolar bone using a high repetition rate transverse excited atmospheric pressure CO2 laser operating at lambda=9.3 micro. J Biomed Opt. 2006;11(6):064008. doi:10.1117/1.2401151.
15. Nevins M, Giannobile WV, McGuire MK, et al. Platelet‐derived growth factor stimulates bone fill and rate of attachment level gain: results of a large multicenter randomized controlled trial. J Periodontol. 2005;76(12):2205-2215. doi:10.1902/jop.2005.76.12.2205.
MORE CLINICAL TIPS FROM DR. SCOTT FROUM . . .
About the Author

Scott Froum, DDS
Editorial Director
Scott Froum, DDS, a graduate of the State University of New York, Stony Brook School of Dental Medicine, is a periodontist in private practice at 1110 2nd Avenue, Suite 305, New York City, New York. He is the editorial director of Perio-Implant Advisory and serves on the editorial advisory board of Dental Economics. Dr. Froum, a diplomate of both the American Academy of Periodontology and the American Academy of Osseointegration, is in the fellowship program at the American Academy of Anti-aging Medicine, and is a volunteer professor in the postgraduate periodontal program at SUNY Stony Brook School of Dental Medicine. He is a trained naturopath and is the scientific director of Meraki Integrative Functional Wellness Center. Contact him through his website at drscottfroum.com or (212) 751-8530.