Beyond bisphosphonates: The top 5 drug classes associated with medication-related osteonecrosis of the jaw (MRONJ)
Key Highlights
- MRONJ is associated with several drug classes beyond bisphosphonates, including RANKL inhibitors, antiangiogenic agents, corticosteroids, chemotherapies, and immunomodulators.
- Denosumab, a RANKL inhibitor, suppresses osteoclast activity and carries MRONJ risk comparable to high-dose IV bisphosphonates.
- Antiangiogenic drugs impair vascular supply needed for bone vitality and mucosal healing, significantly increasing MRONJ risk.
- Corticosteroids and immunosuppressants weaken immunity and impair healing, contributing to osteonecrosis—especially when combined with other risk medications.
- Chemotherapeutic agents disrupt cellular turnover, angiogenesis, and immune function, creating synergistic MRONJ risk when used with antiresorptives.
Medication-related osteonecrosis of the jaw (MRONJ) remains one of the most challenging complications encountered in implant dentistry, periodontics, and oral surgery. Although bisphosphonates have long been recognized as the prototypical culprit, they represent only one subset of systemic medications capable of impairing bone remodeling, angiogenesis, and immune function within the craniofacial skeleton.
Over the past decade, the spectrum of drugs associated with MRONJ has expanded significantly, mirroring advances in oncology, autoimmune disease treatment, and immunomodulation. Importantly, clinicians must recognize that the mechanism of MRONJ is multifactorial—rooted in suppressed osteoclast function, microvascular impairment, dysregulated immunity, and diminished soft-tissue healing.
As patients increasingly receive targeted therapies, implant and periodontal specialists must maintain heightened vigilance regarding risks, drug interactions, and timing of elective oral procedures. This article reviews the top five drug classes beyond bisphosphonates that have demonstrated a clinically significant association with MRONJ, summarizing mechanisms, pharmacologic actions, and implications for treatment planning.
No. 1: RANKL inhibitors (e.g., denosumab)
Although often grouped with bisphosphonates, RANKL inhibitors represent a distinct class of antiresorptive medication with a different pharmacodynamic profile.
Mechanism of action
Denosumab is a monoclonal antibody that binds to the receptor activator of nuclear factor-kappa B ligand (RANKL). By preventing RANKL from activating its receptor (RANK) on osteoclast precursors, denosumab inhibits osteoclast formation, function, and survival.1 Whereas bisphosphonates integrate into the bone matrix with long half-lives, denosumab does not bind bone. Its effects are reversible and time-limited—usually within six months.
Why it causes MRONJ
Despite differences from bisphosphonates, the end result is similar: profound suppression of osteoclastic bone turnover, especially in high-remodeling bones like the mandible.
Contributing mechanisms include:
- Inhibition of normal microdamage repair
- Impaired response to infection
- On-off bone turnover cycles leading to timing complications
Denosumab carries a risk of MRONJ at least comparable to high-dose IV bisphosphonates, especially in oncology dosing (120 mg monthly).
No. 2: Antiangiogenic drugs (e.g., sunitinib, bevacizumab)
Antiangiogenic agents—used extensively in oncology—represent one of the most consistently documented nonantiresorptive drug classes associated with MRONJ.
Mechanism of action
Drugs such as sunitinib and bevacizumab exert their anticancer effects by blocking the formation of new blood vessels through inhibition of vascular endothelial growth factor (VEGF).2
Why they cause MRONJ
Normal angiogenesis is essential for bone vitality and mucosal regeneration. Inhibition of VEGF pathways results in:
- Reduced microvascular perfusion
- Delayed epithelial closure after extractions
- Compromised immune delivery
- Slower integration of grafts
Patients receiving antiangiogenic therapy exhibit MRONJ risk even without concurrent antiresorptives, though risk increases synergistically when therapies overlap.
No. 3: Corticosteroids (e.g., prednisolone, dexamethasone)
Corticosteroids are widely used in autoimmune disorders, pulmonary disease, oncology, and transplant medicine. Their chronic use potentiates MRONJ risk by altering immune, vascular, and bone physiology.3
Mechanism of action
Corticosteroids suppress inflammation and immunity through gene transcription pathways that diminish cytokine signaling.
Why they cause MRONJ
- Corticosteroids contribute to osteonecrosis through:
- Inhibition of osteoblast differentiation
- Promotion of osteocyte apoptosis
- Impaired collagen synthesis
- Immune suppression
- Microvascular changes that reduce perfusion
When combined with antiresorptives or antiangiogenics, corticosteroids significantly amplify MRONJ risk.4
No. 4: Chemotherapy agents (e.g., docetaxel, paclitaxel, everolimus)
Several chemotherapeutic agents have been implicated in MRONJ, particularly taxanes (docetaxel, paclitaxel) and mTOR inhibitors (everolimus).5,6
Mechanism of action
Taxanes inhibit microtubule function, preventing cell division. Everolimus suppresses cellular proliferation and angiogenesis.
Why they cause MRONJ
Chemotherapy contributes to osteonecrosis through:
- Severe immunosuppression
- Mucositis-related ulceration allowing bacterial penetration
- Reduced angiogenesis
- Impaired osteoblastic bone formation
- Increased susceptibility to infection
Chemotherapy’s MRONJ risk becomes clinically significant when combined with antiresorptives.
No. 5: Immunomodulators and immunosuppressants (e.g., methotrexate, adalimumab, etanercept, rituximab)
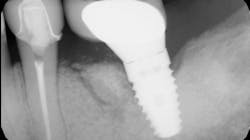
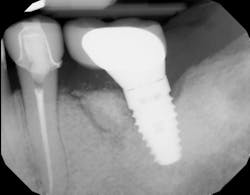
Immunomodulatory medications—used for rheumatoid arthritis, psoriasis, IBD, and lupus—represent a growing contributor to MRONJ (figures 1a and 1b).7,8
Mechanism of action
- Methotrexate suppresses DNA synthesis.
- Adalimumab and etanercept block TNF-α.
- Rituximab depletes CD20+ B cells.
Why they cause MRONJ
These agents disrupt immune function and inflammatory regulation, resulting in:
- Reduced ability to contain periodontal infections
- Impaired macrophage and neutrophil function
- Altered osteoclast–osteoblast coupling
- Secondary reductions in angiogenic signaling
Although MRONJ risk is lower than with antiresorptives, risk increases with combination therapy or chronic high-dose use.
Clinical implications for implant and periodontal therapy
Risk assessment (clinicians should evaluate)
- Current or past antiresorptive use
- Antiangiogenic therapy
- Immunosuppressive regimens
- Corticosteroid exposure
- Chemotherapy cycles
Timing considerations
- Denosumab: schedule surgery near the end of a dosing cycle.
- Chemotherapy: avoid surgery during neutropenia or mucositis.
- Antiangiogenics: consider physician-directed drug holidays when appropriate.
Surgical considerations
High-risk patients may benefit from:
- Atraumatic techniques/primary closure of wound
- Adding biologic regenerative materials to aid in healing
- Antibacterial mouthrinses without alcohol
- Antibiotics when indicated
- Avoiding implants in high-risk zones without clearance
Conclusion
Bisphosphonates remain the best-known drug class associated with MRONJ, but they represent only a small portion of the broader pharmacologic landscape. Denosumab, antiangiogenic drugs, corticosteroids, chemotherapies, and immunomodulators all contribute to MRONJ risk through mechanisms involving impaired angiogenesis, immune suppression, and disrupted bone remodeling. As systemic therapies expand, dental clinicians must strengthen diagnostic vigilance, improve medical screening, and collaborate with physicians to optimize surgical outcomes while minimizing MRONJ risk.
Editor’s note: This article originally appeared in Perio-Implant Advisory, a chairside resource for dentists and hygienists that focuses on periodontal- and implant-related issues. Read more articles and subscribe to the newsletter.
References
- Lee K, Kim K, Kim JY, et al. Mechanisms underlying medication-related osteonecrosis of the jaw. Oral Dis. 2025;31(4):1073-1083. doi:10.1111/odi.15198
- Pimolbutr K, Porter S, Fedele S. Osteonecrosis of the jaw associated with antiangiogenics in antiresorptive-naïve patient: a comprehensive review of the literature. Biomed Res Int. 2018;2018:8071579. doi:10.1155/2018/8071579
- Toriumi S, Kobayashi A, Uesawa Y. Comprehensive study of the risk factors for medication-related osteonecrosis of the jaw based on the Japanese adverse drug event report database. Pharmaceuticals (Basel). 2020;13(12):467. doi:10.3390/ph13120467
- Saad F, Brown JE, Van Poznak C, et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol. 2012;23(5):1341-1347. doi:10.1093/annonc/mdr435
- Teoh L, Moses G, Nguyen AP, McCullough MJ. Medication-related osteonecrosis of the jaw: analysing the range of implicated drugs from the Australian database of adverse event notifications. Br J Clin Pharmacol. 2021;87(7):2767–2776. doi:10.1111/bcp.14681
- Yamamoto D, Tsubota Y, Utsunomiya T, et al. Osteonecrosis of the jaw associated with everolimus: a case report. Mol Clin Oncol. 2017;6(2):255-257. doi:10.3892/mco.2016.1100
- Furudate K, Satake A, Narita N, Kobayashi W. Methotrexate-related lymphoproliferative disorder in patients with osteonecrosis of the jaw: a 3-case report and literature review. J Oral Maxillofac Surg. 2018;76(1):97-111. doi:10.1016/j.joms.2017.05.027
- Henien M, Carey B, Hullah E, Sproat C, Patel V. Methotrexate-associated osteonecrosis of the jaw: a report of two cases. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;124(6):e283-e287. doi:10.1016/j.oooo.2017.09.005
About the Author

Scott Froum, DDS
Editorial Director
Scott Froum, DDS, a graduate of the State University of New York, Stony Brook School of Dental Medicine, is a periodontist in private practice at 1110 2nd Avenue, Suite 305, New York City, New York. He is the editorial director of Perio-Implant Advisory and serves on the editorial advisory board of Dental Economics. Dr. Froum, a diplomate of both the American Academy of Periodontology and the American Academy of Osseointegration, is in the fellowship program at the American Academy of Anti-aging Medicine, and is a volunteer professor in the postgraduate periodontal program at SUNY Stony Brook School of Dental Medicine. He is a trained naturopath and is the scientific director of Meraki Integrative Functional Wellness Center. Contact him through his website at drscottfroum.com or (212) 751-8530.

Stephen Kelleher, DMD
Stephen Kelleher, DMD, is a periodontist practicing in the greater New York City area, originally from Pittsburgh, Pennsylvania. He earned his DMD from Case Western Reserve University in 2022 and completed his periodontics and implant dentistry training at New York University in 2025. Dr. Kelleher has received several awards recognizing his excellence in periodontics and regenerative dentistry. He is dedicated to evidence-based, patient-centered care with a focus on periodontal therapy, oral tissue regeneration, and implant surgery.