Medication-related osteonecrosis of the jaw (MRONJ) consent form
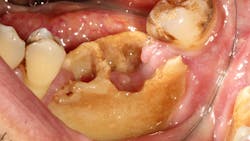
Medication-related osteonecrosis of the jaw (MRONJ) is a severe adverse drug reaction, consisting of progressive bone destruction in the maxillofacial region.1 Once known as osteonecrosis of the jaw (ONJ), ONJ was changed to bisphosphonate-related osteonecrosis of the jaw (BRONJ). Now, it is known as MRONJ because various medications other than bisphosphonate therapy can precipitate this disease.
There are several suggested hypotheses that could explain MRONJ’s unique localization in the jaws: inflammation or infection, microtrauma, altered bone remodeling or oversuppression of bone resorption, angiogenesis inhibition, soft tissue bisphosphonates’ toxicity, peculiar biofilm of the oral cavity, terminal vascularization of the mandible, suppression of immunity, or vitamin D deficiency.
Patients are affected by MRONJ if all the following clinical manifestations are demonstrated:
- Ongoing or antecedent treatment with antiangiogenic or antiresorptive drugs
- No patient history of radiation therapy or manifest metastasis to the jaw
- Exposed bone or presence of an intraoral or extraoral fistula in the maxillofacial region persisting for more than eight weeks
Staging and treatment of MRONJ2
Stage 0: Medical treatment in the form of antiseptic, analgesic, antibiotic, and antiphlogistic therapy should be used, and management of local risk factors is indicated. Low-level laser therapy is a possible choice for treatment of osteonecrosis because of its regenerative possibilities.
Stage 1: If exposed and necrotic bone or fistulae are present, rinsing with antiseptic fluids and covering with an adhesive paste three times a day is indicated. In the absence of healing tendency after eight weeks, a surgical debridement approach may be warranted.
Stage 2: After two weeks of medical therapy to reduce inflammatory symptoms, surgical debridement is indicated. It should be as conservative as possible but extended as much as necessary to ensure a complete removal of the affected bone. Antibiotic and antiphlogistic treatments should be administered.
Stage 3: Marginal or segmental osteotomies are recommended for severe cases. Invasive surgery is indicated only if it could improve the patient’s quality of life. In other cases, or if the patient rejects surgery, a conservative approach to control symptoms and prevent the progression of osteonecrosis is administered.
Importance of screening and treatment
Dental screening and adequate treatment are fundamental to reduce the risk of osteonecrosis in patients under antiresorptive or antiangiogenic therapy, or before initiating the administration. Informed consent and a signed consent form are essential for dental practitioners treating patients who use medications that can precipitate MRONJ.
Download this MRONJ consent form that can be used in the dental practice:
Editor’s note: This article originally appeared in Perio-Implant Advisory, a chairside resource for dentists and hygienists that focuses on periodontal- and implant-related issues. Read more articles and subscribe to the newsletter.
References
- Ruggiero SL, Dodson TB, Fantasia J, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J Oral Maxillofac Surg. 2014;72(10):1938-1956. doi:10.1016/j.joms.2014.04.031
- Rosella D, Papi P, Giardino R, Cicalini E, Piccoli L, Pompa G. Medication-related osteonecrosis of the jaw: clinical and practical guidelines. J Int Soc Prev Community Dent. 2016;6(2):97-104. doi:10.4103/2231-0762.178742
Scott Froum, DDS, a graduate of the State University of New York, Stony Brook School of Dental Medicine, is a periodontist in private practice at 1110 2nd Avenue, Suite 305, New York City, New York. He is the editorial director of Perio-Implant Advisory and serves on the editorial advisory board of Dental Economics. Dr. Froum, a diplomate of both the American Academy of Periodontology and the American Academy of Osseointegration, is a volunteer professor in the postgraduate periodontal program at SUNY Stony Brook School of Dental Medicine. He is a PhD candidate in the field of functional and integrative nutrition. Contact him through his website at drscottfroum.com or (212) 751-8530.
About the Author

Scott Froum, DDS
Editorial Director
Scott Froum, DDS, a graduate of the State University of New York, Stony Brook School of Dental Medicine, is a periodontist in private practice at 1110 2nd Avenue, Suite 305, New York City, New York. He is the editorial director of Perio-Implant Advisory and serves on the editorial advisory board of Dental Economics. Dr. Froum, a diplomate of both the American Academy of Periodontology and the American Academy of Osseointegration, is in the fellowship program at the American Academy of Anti-aging Medicine, and is a volunteer professor in the postgraduate periodontal program at SUNY Stony Brook School of Dental Medicine. He is a trained naturopath and is the scientific director of Meraki Integrative Functional Wellness Center. Contact him through his website at drscottfroum.com or (212) 751-8530.