Why do hormonal IUDs cause gingivitis?
Key Highlights
- Hormonal IUDs releasing progestin can heighten gingival inflammation by lowering immune thresholds, making even minimal plaque trigger exaggerated bleeding and edema.
- Accurate diagnosis requires linking symptom patterns to hormone fluctuations and ruling out other systemic or medication-related causes.
- Targeted home care, shorter periodontal maintenance intervals, and interdisciplinary communication significantly improve outcomes for hormone-sensitive gingivitis.
Gingivitis remains one of the most common inflammatory conditions seen in clinical practice, with some estimates in the range of affecting 90% of the US population, yet its etiology is multifactorial and often influenced by systemic physiology.1 One emerging area of interest is the effect of hormonal intrauterine devices (IUDs) on gingival health. Although IUDs are primarily viewed through a gynecologic lens, the hormonal mechanisms that make these devices effective contraceptives can also alter the immune response within the oral cavity.
Do hormonal IUDs increase gingivitis risk?
Hormonal fluctuations due to progestin release
Hormonal IUDs introduce a continuous, localized release of synthetic progestin. Although systemic levels remain lower than those seen with oral contraceptives, these hormones still exert systemic biologic influence. Progestin mimics progesterone, which is well documented to impact gingival tissue.
Exaggerated immune response in gingival tissues
Progesterone and progestin increase vascular permeability, enhance gingival blood flow, upregulate inflammatory cytokines, and alter neutrophil function. The result is an exaggerated inflammatory response to even low levels of plaque.2
Plaque-induced gingivitis exacerbated by hormonal shifts
Hormonal changes do not cause gingivitis by themselves—plaque remains the etiologic agent. However, when inflammatory thresholds drop due to progesterone imbalance, patients experience more pronounced symptoms.3
Nonhormonal IUDs do not share this risk profile
Copper IUDs avoid progesterone-related effects entirely and do not increase gingivitis risk.
Clinical management recommendations for dental care providers
No. 1: Expand the medical and contraceptive history
A superficial intake form is often inadequate when dealing with hormone-modulated gingival changes.4 Dental providers should ask targeted, open-ended questions to help identify patterns tied to progestin exposure. For example:
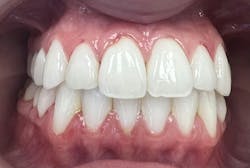
- “Did your gum bleeding begin or worsen after your IUD was placed?” Many patients experience a noticeable shift within one to three months following hormonal IUD insertion (figure 1).
- “Do you notice your gum symptoms fluctuate with your menstrual cycle?” Hormone-sensitive gingivitis often intensifies during the luteal phase, when progesterone peaks.
- “Do you take supplements or medications that affect hormone metabolism?” Vitamin D deficiency, anti-anxiety medications, thyroid disorders, and PCOS can amplify gingival inflammatory responses.
The clinician should document contraception as a modifiable risk factor, similar to smoking or uncontrolled diabetes, when evaluating gingival disease severity and recurrence.
No. 2: Diagnostic criteria and differential diagnosis
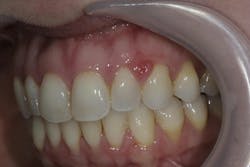
Differentiating the etiology of hormonal gingivitis from other pathologies is essential. A hormone-amplified presentation may include (figure 2):
- “Disproportionate bleeding” where papillae bleed on gentle probing despite low plaque indices.
- Localized papillary inflammation in the absence of calculus, which may mimic early-stage necrotizing gingivitis but lacks ulceration.
- Soft, boggy gingiva due to increased vascular permeability and edema related to progesterone activity.
- Flare-ups that coincide with systemic hormone fluctuations, including cycle changes or stress-induced endocrine shifts.
Differential diagnosis should rule out:
- Leukemia-associated gingival enlargement
- Medication-induced gingival hyperplasia
- Vitamin C deficiency
- Early-stage periodontitis masked by hormonal edema
If tissue does not respond predictably to mechanical therapy, hormone involvement should be strongly suspected.
No. 3: Enhanced home care protocols
Hormonal gingivitis is uniquely responsive to improved oral hygiene—even more so than conventional plaque-driven gingivitis—because reducing biofilm reduces the trigger that amplifies the hormonal inflammatory cascade.
Specific recommendations include:
- Power toothbrushes: Oscillating–rotating brushes have shown superior plaque removal in patients with inflammation driven by lower immune thresholds.
- Interdental brushes: These outperform traditional floss for patients with papillary inflammation, as the physical contact scrubs more biofilm from the concavities.
- Water flossers: Water flossers are particularly beneficial for hormonally sensitive patients who experience bleeding anxiety. Water pressure reduces biofilm without triggering mechanical bleeding.
- Anti-inflammatory rinses: Essential oil rinses can reduce gingival inflammation by modulating biofilm complexity.
- Short-term chlorhexidine: Use chlorhexidine as a two-week pulse during inflammatory flares.
- Xylitol gum or lozenges: These reduce pathogenic bacterial adherence and elevate salivary buffering capacity.
Patients should be reassured repeatedly that bleeding does not mean they are brushing incorrectly, which is a common misconception among hormone-sensitive individuals.
No. 4: Periodontal maintenance frequency
Hormonal IUD users often benefit from being treated similarly to patients with chronic inflammatory disorders such as diabetes or autoimmune conditions. This means:
- Two-to-three-month intervals reduce the microbial load before the inflammatory flare cycle reinstates.
- Targeted debridement focuses on papillary regions most responsive to progesterone.
- Subgingival antimicrobial irrigation—such as chlorhexidine, betadine, or hydrogen peroxide—suppresses bacterial load long enough for tissues to stabilize.
- Biofilm testing (if available) helps track whether hormone-triggered symptoms correspond with bacterial shifts.
Patients should be informed that shorter intervals are part of a medically justified, evidence-based risk mitigation plan, not “extra cleanings.”
No. 5: Interprofessional collaboration
Gynecologists and primary care physicians often appreciate communication when dental inflammation persists despite mechanical therapy.5 Effective collaboration may include:
- Reporting documented gingival inflammation with periodontal charting
- Describing onset correlated with IUD placement
- Asking whether alternative hormonal formulations exist with lower systemic progesterone exposure
- Discussing whether the patient has reported other systemic side effects such as headaches, breast tenderness, mood changes, or acne—each suggesting heightened progestin sensitivity
This communication often helps the physician determine whether the patient is among the small but clinically notable group who may benefit from modifying their contraceptive strategy.
No. 6: Expanded patient education strategies
Patients experiencing hormone-amplified gingivitis often worry they are experiencing the start of periodontal disease. Reassure them by explaining:
- Progestin increases blood flow and vascular permeability, which exaggerates the appearance of inflammation.
- Inflammation typically improves within weeks of improved hygiene and supportive care.
- Copper IUDs do not cause these changes, so contraceptive choice matters.
- Consistency is more important than perfection—small lapses can trigger noticeable symptoms due to hormone sensitivity.
Written home-care instructions and visual biofilm-disclosing demonstrations help reinforce understanding.
No. 7: Managing refractory gingivitis (gingivitis that returns after treatment)
For patients with persistent symptoms:
- Evaluate vitamin D levels, as deficiency is strongly associated with chronic gingival inflammation and hormone dysregulation.
- Use salivary inflammatory biomarker testing to evaluate neutrophil activity and cytokine expression.
- Prescribe steroid rinses or gels for short-term anti-inflammatory control.
- Evaluate for systemic endocrine disorders such as thyroid dysfunction, PCOS, adrenal dysregulation, and insulin resistance.
- Nonsurgical periodontal therapy should be considered when inflammation leads to early attachment loss.
These patients may require periodic anti-inflammatory intervention due to persistent progesterone responsiveness.
No. 8: Long-term monitoring
Long-term monitoring should focus on:
- Bleeding index trends
- Papillary inflammation patterns
- Pocket depths and early clinical attachment changes
- Patient-reported cycle-related flares
- Radiographic changes in areas of chronic inflammation
Tracking patterns helps distinguish stable hormone-sensitive gingivitis from incipient periodontitis, which requires more aggressive intervention.
Conclusion
Managing gingival inflammation in patients using hormonal IUDs requires a layered, multidisciplinary approach that goes far beyond standard oral hygiene counseling. Because progestin alters vascular physiology, inflammatory thresholds, and immune modulation, these patients often experience gingival responses that appear disproportionate to local plaque levels. The recommendations provide a deeper clinical understanding of how to diagnose, manage, and minimize hormone-amplified gingival inflammation.
Editor’s note: This article originally appeared in Perio-Implant Advisory, a chairside resource for dentists and hygienists that focuses on periodontal- and implant-related issues. Read more articles and subscribe to the newsletter.
References
- Gasner NS, Schure RS. Periodontal disease. In: StatPearls [Internet]. StatPearls Publishing; 2025. https://www.ncbi.nlm.nih.gov/books/NBK554590/
- Singh P, Dev YP, Kaushal S. Progesterone supplementation – beware of changes in the oral cavity. J Hum Reprod Sci. 2013;6(2):165. doi:10.4103/0974-1208.117163
- Carranza FA, Newman M. Clinical Periodontology. 13th ed., Elsevier; 2019.
- Jawed STM, Tul Kubra Jawed K. Understanding the link between hormonal changes and gingival health in women: a review. Cureus. 2025;17(6):e85270. doi:10.7759/cureus.85270
- Rojo MG, Lloret MRP, Gironés JG. Oral manifestations in women using hormonal contraceptive methods: a systematic review. Clin Oral Investig. 2024;28(3):184. doi:10.1007/s00784-024-05573-x
About the Author

Scott Froum, DDS
Editorial Director
Scott Froum, DDS, a graduate of the State University of New York, Stony Brook School of Dental Medicine, is a periodontist in private practice at 1110 2nd Avenue, Suite 305, New York City, New York. He is the editorial director of Perio-Implant Advisory and serves on the editorial advisory board of Dental Economics. Dr. Froum, a diplomate of both the American Academy of Periodontology and the American Academy of Osseointegration, is in the fellowship program at the American Academy of Anti-aging Medicine, and is a volunteer professor in the postgraduate periodontal program at SUNY Stony Brook School of Dental Medicine. He is a trained naturopath and is the scientific director of Meraki Integrative Functional Wellness Center. Contact him through his website at drscottfroum.com or (212) 751-8530.

Stacy Spizuoco, DDS
Stacy Spizuoco, DDS, is a graduate of New York University College of Dentistry. She works in private practice in New York City and has volunteered as a clinical instructor at Columbia College of Dental Medicine. Dr. Spizuoco performs charitable work and volunteers at local missions, is a member of the American Dental Association, a fellow of the American College of Dentists, a board member for various dental organizations, a member of the American Academy of Clear Aligners, and a KOL for Glidewell Dental Lab.