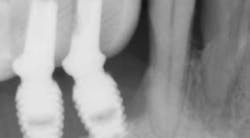
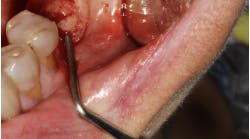
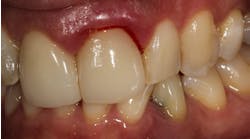
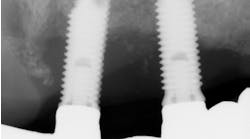
Which is most effective for the treatment of peri-implantitis: Mechanical, chemical, or laser?
Treatment varies significantly—from nonsurgical therapy with or without adjunctive local-release antibiotics to surgeries that include flap debridement procedures with or without osseous resection and implantoplasty. Regenerative procedures are also available, such as bone grafts (or bone substitutes) with or without barrier membranes. (7) However, because of the heterogeneity and many variables in peri-implantitis studies included in systematic reviews and meta-analysis, the authors of two of the more complete reviews could not identify any one protocol as being more predictable or superior than the others in terms of treatment outcomes. (8, 9) A more recent literature review of successful surgical protocols for the treatment of peri-implantitis similarly concluded that more long-term comparative studies are necessary to evaluate the superiority of one treatment over another. (10)
Regardless of the treatment, however, both clinicians and researchers agree that an essential part of any treatment protocol for peri-implantitis must include decontamination of the exposed contaminated implant surface. Here, too, systemic evaluations of mechanical, chemical, and laser detoxification have concluded that due to the variety of surface decontamination treatments with limited comparative studies, no specific protocol has been shown to be more effective than the others. (11, 12)
Recently, a regenerative surgical protocol that uses both mechanical and chemical means for implant surface detoxification—together with a bone graft and/or substitute including coverage with a barrier membrane and a two- to 10-year follow-up period—has been shown to have a 98.8% implant survival rate. The results of this study were based on peri-implantitis treatment of 170 consecutive dental implants in 100 patients. (13) In that specific study, an air powder abrasive was utilized as part of the surface decontamination.
Several other studies have suggested the adjunctive use of various lasers—CO2, Er:YAG, Nd:YAG, and Er,Cr:YSGG—to detoxify contaminated implant surfaces as part of a regenerative protocol to treat peri-implantitis. (14, 15) Two systematic literature reviews have also reported the possible advantages of using lasers in the treatment of peri-implantitis. (16,17) The former suggested that “adjunctive use of a laser disinfection protocol with GTR [guided tissue regeneration] for the treatment of peri-implantitis may improve clinical and radiographic findings up to five years after therapy." (16) However, both reviews concluded that “no firm conclusion can be drawn at this moment because of limited sample sizes and short follow-up periods.” Moreover, the reviews also agreed that "there is a need for more well-designed, longitudinal, randomized controlled clinical trials.” (17)
Based on the long-term success with regenerative protocols that did not use adjunctive lasers in their treatments, the potential use of the various lasers should be evaluated and studied to determine the advantages and disadvantages of their use and compare clinical outcomes when they are or are not used.
References
1. Lindhe J, Meyle J, Group D of European Workshop on Periodontology. Peri-implant diseases: consensus report of the sixth European workshop on periodontology. J Clin Periodontol. 2008;35(Suppl 8):282-285. doi: 10.1111/j.1600-051X.2008.01283.x.
2. Sanz M, Chapple IL, Working Group 4 of the VIII European Workshop on Periodontology. Clinical research on peri-implant diseases: consensus report of working group 4. J Clin Periodontol. 2012;39(Suppl 12):202-206. doi: 10.1111/j.1600-051X.2011.01837.x.
3. Mombelli A, van Oosten MA, Schurch E Jr, Land NP. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol Immunol. 1987;2(4):145-151.
4. Augthun M, Conrads G. Microbial findings of deep peri-implant bone defects. Int J Oral Maxillofac Implants. 1997;12(1):106-112.
5. Salcetti JM, Moriarty JD, Cooper LF, et al. The clinical, microbial, and host response characteristics of the failing implant. Int J Oral Maxillofac Implants. 1997;12(1):32-42.
6. Linde J, Meyle J, Group D of European Workshop of Periodontology. Peri-implant diseases: consensus report of the sixth European workshop of Periodontology. J Clin Periodontol. 2008;35(Suppl 8):286-291.
7. Okayasu K, Wang HL. Decision tree for the management of peri-implant diseases. Implant Dent. 2012;21(3):253.
8. Heitz-Mayfield LJ, Mombelli A. The therapy of peri-implantitis: a systematic review. Int J Oral Maxillofac Implants. 2014;(Suppl):325-345. doi: 10.11607/jomi.2014suppl.g5.3.
9. Chan HL, Lin GH, Suarez F, MacEachern M, Wang HL. Surgical management of peri-implantitis: a systematic review and meta-analysis of treatment outcomes. J Periodontol. 2014;85(8):1027-1041. doi: 10.1902/jop.2013.130563.
10. Froum SJ, Dagba AS, Shi Y, Perez-Asenjo A, Rosen PS, Wang WC. Successful surgical protocols in the treatment of peri-implantitis: a narrative review of the literature. Implant Dent. 2016;25(3):416-426. doi: 10.1097/ID.0000000000000428.
11. Suarez F, Monje A, Galindo-Moreno P, Wang HL. Implant surface detoxification: a comprehensive review. Implant Dent. 2013;22(5)465-473. doi: 10.1097/ID.0b013e3182a2b8f4.
12. Chan HL, Lin GH, Suarez F, MacEachern M, Wang HL. Surgical management of peri-implantitis: a systematic review and meta-analysis of treatment outcomes. J Periodontol. 2014;85(8):1027-1041. doi: 10.1902/jop.2013.130563.
13. Froum SJ, Froum SH, Rosen PS. A regenerative approach to the successful treatment of peri-implantitis: a consecutive series of 170 implants in 100 patients with 2- to 10-year follow-up. Int J Periodontics Restorative Dent. 2015;35(6)857-863. doi: 10.11607/prd.2571.
14. Norton MR. Efficacy of Er:YAG laser in the decontamination of peri-implant disease: a one-year prospective closed cohort study. Int. J Periodontics Restorative Dent. 2017;37(6):781-788. doi: 10.11607/prd.3324.
15. Nevins M, Nevins ML, Yamamoto A, et al. Use of Er:YAG laser to decontaminate infected dental implant surface in preparation for reestablishment of bone-to-implant contact. Int J Periodontics Restorative Dent. 2014;34(4):461-466. doi: 10.11607/prd.2192.
16. Geisinger ML, Holmes CM, Vassilopoulos PJ, Geurs NC, Reddy MS. Is laser disinfection an effective adjunctive treatment to bone augmentation for peri-implantitis? A review of current evidence. Clin Adv Periodontics. 2014;4(4):274-279. doi: 10.1902/cap.2013.130048.
17. Natto ZS, Aladmawy M, Levi PA Jr, Wang HL. Comparison of the efficacy of different types of lasers for the treatment of peri-implantitis: a systematic review. Int J Oral Maxillofac Implants. 2015;30(2):338-345. doi: 10.11607/jomi.3846.