Contaminated dental implants: Debris in the internal chamber
New implants may be sterile but not clean when packed.
Introduction
Ensuring the quality of medical devices, including dental implants, is paramount to their effectiveness and safety and must adhere to a series of rules.1-4 Manufacturers often emphasize meticulous processes to achieve surfaces conducive to osseointegration, highlighting their products as impeccably machined, cleaned, and sterilized.5-7 However, while external contamination levels are increasingly assessed,8-13 research on the cleanliness of internal features, such as the internal chamber of the dental implant, remains limited.
This article describes a study that found all the dental implants examined were contaminated with titanium debris within the internal aspect of the implant fixture when visually inspected at 30x magnification and swabbed.
Galling
Titanium (Ti) and its alloys are notoriously susceptible to galling, defined by the American Society for Testing and Materials (ASTM) as “a form of surface damage arising between sliding solids, distinguished by macroscopic, usually localized, roughening and creation of protrusions above the original surface. It often includes plastic flow or material transfer or both.”14
As such, Ti is considered difficult to machine and poorly suited to many tribological applications (factors that involve friction, lubrication, and wear) such as dental implants and their inside screw threads.15 Ti chip formation (particles) is known to occur, and these particles may remain unless effectively removed.16 Another factor limiting the ability to have implants free of debris relates to the removal of debris within the inside of the implant.
Ti particle contamination and debris within these areas can increase screw thread friction and compromise the mating surfaces, resulting in an increased likelihood of screw loosening, considered a common problem.17-19
Biological success may also be dependent upon adequate removal of all Ti particles from within the implant, as these may be inadvertently released within the implant surgical site. Several studies have alluded to the issue of immune responses and a potential cause of peri-implantitis, especially related to fine particulate matter.20-25
Study materials and methods
Seven brand-new, sealed implants from various manufacturers were utilized, with details provided in Table 1.
After removing external packaging, the implants were kept in their carriers to minimize handling. A microscope (SM-3T, AmScope) with photographic capability was employed to examine the connection site and deeper within the internal chamber screw channel shaft down to its base. Any evidence of foreign matter was recorded, and photographs were taken.
To test the internal aspect of the implant, a dry 0.5 cm x 3 cm strip of 3-micron filtration paper was carefully rolled into a fine cone (for smaller sizes, an endodontic paper cone size 60 was used). The cones were inspected and photographed prior to sampling to confirm they were free of contamination. To collect debris, the paper cones were inserted and then rotated three times clockwise and counterclockwise. Once removed, the filter paper cones were examined under 30x magnification and recorded.
Study results
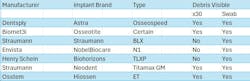
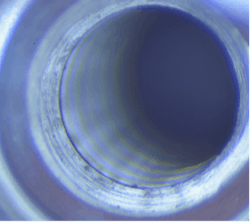
Of the seven major dental implant brands inspected, four contained visible material under magnification (57%), primarily located within the screw channel and connection (figure 1). Debris was also seen in the base of the screw channel in another brand-new implant (figure 2).
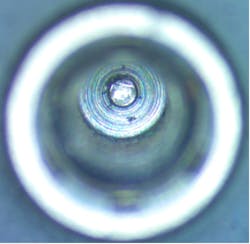
Figure 3a shows a cleaned, rolled swab prior to insertion into the implant screw channel. Figure 3b shows the outcome of this swab.
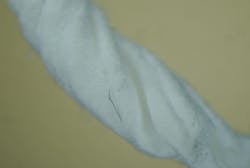
In total, seven out of seven samples yielded foreign material when swabbed (100%), resembling Ti particles and machine shavings. The particle size was estimated to be approximately 1–2 mm for larger shards (figures 3c and 3d) and around 20–30 microns for smaller particles (figure 4).
Clinical implications of contaminated implants
Screw loosening: Keeping screw threads and abutment connections free of debris is crucial to prevent mechanical failures such as abutment screw loosening, a prevalent issue in single-implant crowns.17-19 Inadequate cleaning after thread machining partly due to poor access within the implant can lead to debris accumulation, increased friction, and compromised preload, potentially causing complications.
Screw loosening is considered the most common restorative complication, especially when considering single-unit restorations. The literature cites a range of screw-loosening events up to 12% in single-implant crowns.18 Several factors have been cited that contribute to this issue, one being the effect of friction within different sites of the implant-abutment connection.19 When screw threads contain debris, an alteration in the friction during torque of the screw can occur, which absorbs energy that could otherwise contribute to preload. Further, any debris in the connection site may prevent the abutment from completely seating.
Interestingly, several implant companies advocate the use of laboratory screws during fabrication of the implant restoration presumably to limit contamination on the definitive screw. However, if the inside of the implant has debris, this in part defeats the objective.
Peri-implantitis and implant failure: Ti particles not only pose mechanical risks but also potential biological concerns.20-24 Recently, the impact of Ti particles and their association with inflammatory reactions causing bone resorption and implant rejection have been discussed. Foreign-body reactions may be the primary cause or at least a contributing factor to peri-implant disease.20 The underlying risk factors and detailed pathogenesis of peri-implantitis remain to be elucidated; however, Ti-based implants are known to release Ti particles into the surrounding tissue. The concentration of these particles has been shown to increase significantly at peri-implantitis sites, suggesting that Ti particles may be a potential risk factor for the condition.21,22
Studies investigating the association of Ti particles with peri-implantitis have found that the Ti is mostly found inside epithelial cells, connective tissue, macrophages, and bone. It has been demonstrated that Ti particles derived from the wear of orthopedic implant surfaces can activate macrophages to secrete cytokines and stimulate osteoclastic bone resorption, causing osteolysis.23
Although various mechanisms have been considered responsible for Ti release—including friction during implant insertion, corrosion of the implant surface, friction at the implant-abutment interface, etc.—the internal machining debris is not one of them.24 If and how the metal implant particles are released also remains to be seen. It is possible that during placement of implant components, or washing and drying the site with air, these particles may be ejected into the surrounding tissues. These reactions combine to produce cellular responses with a shift in the delicate balance between the osteoblast and the osteoclast, resulting in bone resorption.25
Products being developed to clean inside chamber of dental implants
Care must be taken when cleaning out the inside of dental implants to prevent the migration of materials into the surroundings. Paper points and suction directed into the inside of the implant are recommended. Novel cleaning systems are also being developed by the company ImplantWise for the purpose of trapping and removing the metal particles.26
Conclusion
All implants evaluated in the study contained debris within the internal aspects, indicating inadequate attention to cleanliness by manufacturers. This underscores the need for improved cleaning methods and stricter quality control measures in dental implant production.
Limitations of this study include the small sample size and the need for further investigation into the nature of the debris found. Methods that remove all debris present within the internal aspect of the implant should be developed by the industry for use in the dental clinic.
As Professor Thomas Albrektsson states: “The weak point is, of course, that it is difficult to state whether a certain ionic or other remnant on implant surfaces really is dangerous or not, at the same time as I assume we all would prefer ‘clean implants.’”
Conflict of interest: Dr. Chandur Wadhwani has a financial interest in implantwisedental.com.
Editor’s note: This article originally appeared in Perio-Implant Advisory, a chairside resource for dentists and hygienists that focuses on periodontal- and implant-related issues. Read more articles and subscribe to the newsletter.
References
- Santos ICT, Gazelle GS, Rocha LA, Tavares JMRS. Medical device specificities: opportunities for a dedicated product development methodology. Expert Rev Med Devices. 2012;9(3):299-311. doi:10.1586/erd.12.3
- Maisel WH. Medical device regulation: an introduction for the practicing physician. Ann Intern Med. 2004;140(4):296-302. doi:10.7326/0003-4819-140-4-200402170-00012
- Dental endosseous implants: an update. ADA Council on Scientific Affairs. J Am Dent Assoc. 1996;127(8):1238-1239.
- Schumann D. FDA and ADA evaluation of dental implants. J Public Health Dent. 1992;52(6):373-374. doi:10.1111/j.1752-7325.1992.tb02308.x
- How it’s made: Alpha Dent Dental Implants. Alpha Dent Implants GmbH. YouTube. August 15, 2017. Accessed March 3, 2024. https://www.youtube.com/watch?v=QJpD1iyFfYw
- Tornos CT – Swiss-type lathe. TornosGroup. YouTube. November 28, 2013. https://www.bing.com/videos/riverview/relatedvideo?q=youtube%20how%20its%20made%20dental%20implants&mid =EB1121C09506A47E8A60EB1121C09506A47E8A60&ajaxhist=0
- Lab tour – implant manufacturing. Glidewell. YouTube. December 13, 2017. https://www.bing.com/videos/riverview/relatedvideo?q=youtube%20how%20its%20made%20dental%20implants&mid =83BE3FB291DFAD16ED4883BE3FB291DFAD16ED48&ajaxhist=0
- Schupbach P, Glauser R, Bauer S. Al2O3 particles on titanium dental implant systems following sandblasting and acid-etching process. Int J Biomater. 2019;2019:6318429. doi:10.1155/2019/6318429
- Abdulla MA, Hasan RH, Al-Hyani OH. Impact of Er,Cr:YSGG laser, sandblast, and acid etching surface modification on surface topography of biodental titanium implants. J Lasers Med Sci. 2023;14:e38. doi:10.34172/jlms.2023.38
- Mtanis T, Biadsee A, Ormianer Z. Assessing the cleanliness of dental implants using scanning electron microscopy and energy-dispersive x-ray spectroscopy analysis–a SEM and EDS in vitro study. J Funct Biomater. 2023;14(3):172. doi:10.3390/jfb14030172
- Duddeck DU, Albrektsson T, Wennerberg A, Larsson C, Beuer F. On the cleanliness of different oral implant systems: a pilot study. J Clin Med. 2019;8(9):1280. doi:10.3390/jcm8091280
- Ganz SD, Duddeck DU, Kurtzman GM. Peri-implantitis and the effect of the implant surface at placement. Compend Contin Educ Dent. 2023;44(1):52-55.
- Albrektsson T, Wennerberg A. On osseointegration in relation to implant surfaces. Clin Implant Dent Relat Res. 2019;21(Suppl 1):4-7. doi:10.1111/cid.12742
- Standard terminology relating to wear and erosion. ASTM International. Updated November 11, 2022. https://www.astm.org/g0040-22a.html
- Wiklund U, Hutchings IM. Investigation of surface treatments for galling protection of titanium alloys. Wear. 2001;250(1–12):1034-1041. doi:10.1016/S0043-1648(01)00730-X
- Xi Y, Bermingham M, Wang G, Dargusch M. Finite element modeling of cutting force and chip formation during thermally assisted machining of Ti6Al4V alloy. J Manuf Sci Eng. 2013;135(6):188-197. doi:10.1115/1.4025740
- Adawi HA, Dewan H, Khawaji A, et al. Effects of blood contamination and decontamination protocol on reverse torque value of abutment screws in dental implants: an in vitro study. Biomimetics (Basel). 2023;8(2):157. doi:10.3390/biomimetics8020157
- Goodacre BJ, Goodacre SE, Goodacre CJ. Prosthetic complications with implant prostheses (2001-2017). Eur J Oral Implantol. 2018;11(Suppl 1):S27-S36.
- Huang Y, Wang J. Mechanism of and factors associated with the loosening of the implant abutment screw: a review. J Esthet Restor Dent. 2019;31(4):338-345. doi:10.1111/jerd.12494
- Jensen OT, Albrektsson T, Jemt T. Foreign body osseointegration. Int J Oral Maxillofac Implants. 2023;38(5):838-840. doi:10.11607/jomi.10694
- Chen L, Tong Z, Luo H, Qu Y, Gu X, Si M. Titanium particles in peri-implantitis: distribution, pathogenesis and prospects. Int J Oral Sci. 2023;15(1):49. doi:10.1038/s41368-023-00256-x
- Safioti LM, Kotsakis GA, Pozhitkov AE, Chung WO, Daubert DM. Increased levels of dissolved titanium are associated with peri-implantitis – a cross-sectional study. J Periodontol. 2017;88(5):436-442. doi:10.1902/jop.2016.160524
- Xing Z, Schwab LP, Alley CF, Hasty KA, Smith RA. Titanium particles that have undergone phagocytosis by macrophages lose the ability to activate other macrophages. J Biomed Mater Res B Appl Biomater. 2008;85(1):37-41. doi:10.1002/jbm.b.30913
- Suárez-López Del Amo F, Garaicoa-Pazmiño C, Fretwurst T, Castilho RM, Squarize CH. Dental implants-associated release of titanium particles: a systematic review. Clin Oral Implants Res. 2018;29(11):1085-1100. doi:10.1111/clr.13372
- Albrektsson T, Canullo L, Cochran D, De Bruyn H. "Peri-implantitis": a complication of a foreign body or a man-made “disease.” Facts and fiction. Clin Implant Dent Relat Res. 2016;18(4):840-849. doi:10.1111/cid.12427
- ImplantWise Dental Innovations. https://implantwisedental.com/
Chandur P.K. Wadhwani, BDS, MSD, is in private practice limited to prosthodontics in Bellevue, Washington. He is an affiliate assistant professor in the department of restorative dentistry at the University of Washington School of Dentistry in Seattle, Washington; associate professor at the University of Oregon Health Science University in Portland, Oregon; and assistant professor of the advanced education program in prosthodontics at Loma Linda University in Loma Linda, California.
Tomas Albrektsson, MD, PhD, RCPSG, is a professor in the department of biomaterials at the University of Gottenburg, Sweden.
Henry H. Rowshan, DDS, is in private practice limited to oral, maxillofacial, and implant surgery in Bellevue, Washington.
Neal C. Raval, BDS, MSD, is in private practice limited to periodontics and implants in Bellevue, Washington.
Kwok-Hung Chung, DDS, PhD, MS, is a professor in the department of restorative dentistry at the University of Washington School of Dentistry in Seattle, Washington.
About the Author
Chandur P.K. Wadhwani, BDS, MSD
Chandur P.K. Wadhwani, BDS, MSD, is in private practice limited to prosthodontics in Bellevue, Washington. He is an affiliate assistant professor in the department of restorative dentistry at the University of Washington School of Dentistry in Seattle, Washington; associate professor at the University of Oregon Health Science University in Portland, Oregon; and assistant professor of the advanced education program in prosthodontics at Loma Linda University in Loma Linda, California.
Updated April 21, 2023

Tomas Albrektsson, MD, PhD, RCPSG
Tomas Albrektsson, MD, PhD, RCPSG, is a professor in the department of biomaterials at the University of Gottenburg, Sweden.
Updated April 2, 2024
Henry H. Rowshan, DDS
Henry H. Rowshan, DDS, is in private practice limited to oral, maxillofacial, and implant surgery in Bellevue, Washington.
Updated April 2, 2024
Neal C. Raval, BDS, MSD
Neal C. Raval, BDS, MSD, is in private practice limited to periodontics and implants in Bellevue, Washington.
Updated April 2, 2024

Kwok-Hung Chung, DDS, PhD, MS
Kwok-Hung Chung, DDS, PhD, MS, is a professor in the department of restorative dentistry at the University of Washington School of Dentistry in Seattle, Washington.
Updated April 2, 2024